Industry News
Industry News in AQ Trials

Industry News
Transforming CAPA Management in Clinical Trials Research For Enhanced Quality and Efficiency
Blog 18/12/2023
Ensuring the integrity and reliability of data is highly crucial yet challenging in clinical trials. Anyhow, with Corrective and Preventive Action (CAPA) management, it becomes seamless to maintain quality and compliance in real-time.
Let’s examine the limitations of traditional CAPA management in clinical trials while proposing innovative solutions for a more effective system.
AQ

Industry News
E-Delegation in Clinical Trials: Overcoming Traditional Challenges
Blog 12/12/2023
Increasingly, the shift from traditional methods to digital innovation is marking a significant turning point in clinical trials. ‘E-delegation’ is one such innovation, which encapsulates the transition from paper-based delegation logs to electronic systems. This shift is more than a mere technological update. It revolutionises how tasks are assigned, monitored, and recorded in clinical trials.
AQ

Industry News
Who Uses CTMS Solutions and Why?
Blog 06/12/2023
The implementation of cutting-edge tools is transforming the way professionals manage and conduct clinical trials, right? CTMS is at the forefront of this technological revolution. From pharmaceutical companies to academic institutions and research organisations, everyone uses CTMS solutions.
AQ

Industry News
What is CAPA Management Software in Clinical Research?
Blog 02/12/2023
CAPA management software is a go-to solution for streamlining clinical trial research processes. It offers a useful, systematic approach that enhances research project quality through proactive issue resolution, preventive measures, and data integrity maintenance. All for better, compliant workflows.
AQ

Industry News
How Long Do Clinical Trials Take?
Blog 28/11/2023
Objective Response Rate (ORR) in clinical trials is the proportion of patients demonstrating either a partial response (PR) or a complete response (CR) to a specific treatment. It excludes stable disease and provides a direct quantitative measure of the treatment’s ability to induce a meaningful reduction or elimination of tumours.
AQ

Industry News
How Long Do Clinical Trials Take?
Blog 28/11/2023
Objective Response Rate (ORR) in clinical trials is the proportion of patients demonstrating either a partial response (PR) or a complete response (CR) to a specific treatment. It excludes stable disease and provides a direct quantitative measure of the treatment’s ability to induce a meaningful reduction or elimination of tumours.
AQ

Industry News
How Long Do Clinical Trials Take?
Blog 24/11/2023
According to Cancer Research UK, the timeline for drug testing and approval varies widely. There’s no fixed duration, but completing all phases of clinical trials, even with the help of a CTMS, may take 10 to 15 years or more. This timeline includes licence issuance as well.
AQ

Industry News
What are the 4 Phases of Clinical Trials?
Blog 23/11/2023
In clinical trial research management, the Clinical Trial Management System (CTMS) and the Electronic Trial Master File (eTMF) play distinct yet important roles. CTMS focuses on operational efficiency—overseeing site management and subject recruitment. In contrast, eTMF is an electronic repository—emphasizing document integrity, version control, and audit trails. All in real-time.
AQ

Industry News
What are the 4 Phases of Clinical Trials?
Blog 22/11/2023
The clinical trial research is divided into four distinct phases, each serving a specific purpose in the pursuit of safe and reliable medicines. These phases constitute a structured journey, guiding investigational products from their initial introduction to thorough evaluation and eventual widespread usage. All of this is effectively planned and executed with the help of a clinical trial management system (CTMS).
AQ
Industry News
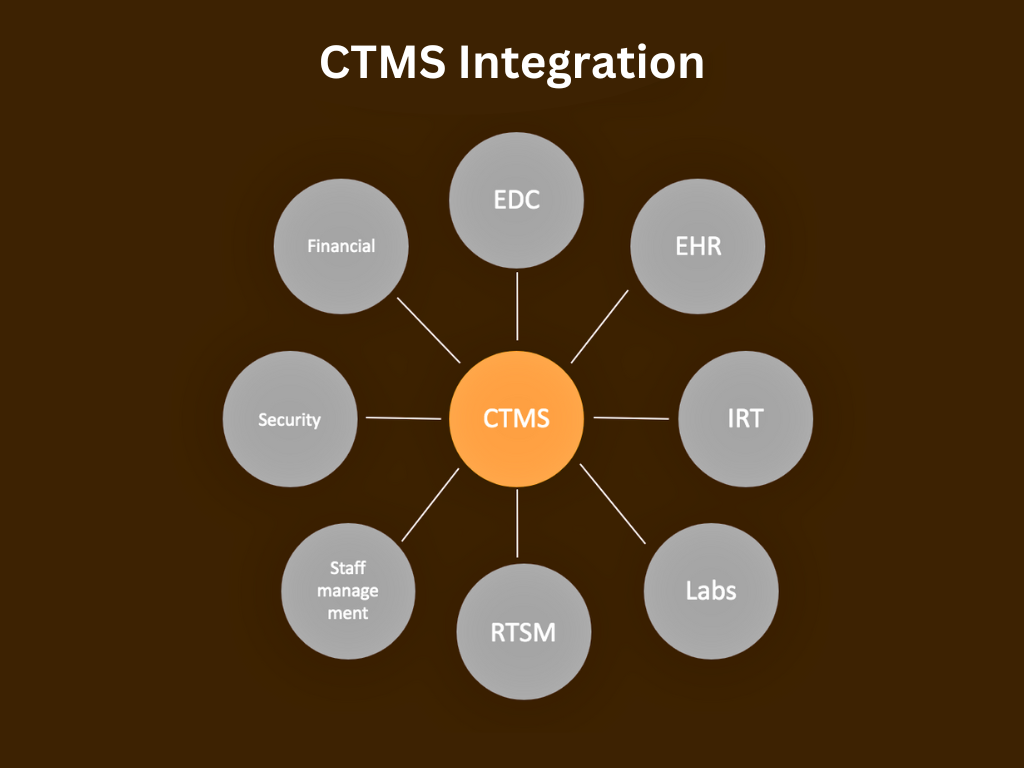
5 Tips for a Successful Integration of CTMS with EDC
Blog 18/11/2023
Clinical Trial Management Systems (CTMS) and Electronic Data Capture (EDC) systems play crucial roles in the efficient conduct of clinical trials. Integrating these systems can streamline processes, improve data quality, and enhance overall trial management. Here are some tips for successful integration of CTMS with EDC
AQ

Industry News
How to Implement and Use CTMS?
Blog 17/11/2023
The successful implementation and utilisation of a CTMS demand strategic planning and execution backed by ongoing adaptation, feedback loops, and a commitment to leveraging technology for the betterment of medical research. This comprehensive guide will walk you through the key steps involved in implementing and effectively using a CTMS, ensuring a seamless and efficient clinical trial management process.
AQ

Industry News
What are Decentralized Clinical Trials: Benefits, Challenges, and Implementation
Blog 16/11/2023
Decentralized clinical trials (DCTs), also known as remote or virtual clinical trials, represent a “modern approach” to conducting clinical research in real-time. Over the past few years, Decentralized Clinical Trials (DCTs) have transformed from a promising innovation to a pivotal force shaping the landscape of clinical research. The future of clinical trials is undeniably decentralized, as it offers a more inclusive and streamlined approach to advancing medical research.
AQ

Industry News
CTMS VS EDC – What’s their Role and Difference in Clinical Research?
Blog 04/11/2023
Clinical trial management systems (CTMS) and electronic data capture (EDC) software are designed to simplify and automate the intricate processes of clinical research. CTMS focuses on the management and coordination of the trial’s administrative aspect — whereas EDC software is dedicated to the collection and management of patient data. Both serve different but complementary roles, ensuring efficiency, accuracy, and success.
AQ

Industry News
7 Reasons Clinical Researchers Need to Use CTMS
Blog 13/10/2023
Clinical Trial Management Systems (CTMS) serve as the backbone of clinical trial research progress. Basically, CTMS is a sophisticated software tool which acts as the guiding force behind clinical trials. It’s a catalyst for ensuring efficient and effective trials. Think of it as the conductor that orchestrates the intricate symphony of clinical research.
In this article, we’ll unravel the significance of CTMS in modern healthcare. Let’s explore why CTMS is a game-changer—ensuring smoother trials and, ultimately, contributing to improved healthcare outcomes.
AQ

Industry News
What is EDC in Clinical Trials?
Blog 13/10/2023
Electronic Data Capture (EDC) emerged as a transformative tool for clinical researchers, ensuring ultimate precision and efficiency. It represents a pivotal shift from traditional paper-based data collection methods to a digital approach, which is better and streamlined. Basically, it enhances the quality, speed, and accuracy of clinical trials in real time.
Let’s get into the ins and outs of EDC in clinical trials—exploring its significance, key features, advantages, and challenges.
AQ

Industry News
What is an eTMF in Clinical Trial Research?
Blog 05/10/2023
eTMF (stands for electronic Trial Master File) is leveraged for the management of clinical trials in the pharmaceutical, biotechnology, and medical device industries. It offers advantages in terms of efficiency, accessibility, security, and regulatory compliance compared to traditional paper-based TMFs. Ultimately, it improves the conduct and documentation of clinical trials.
Let’s find out more about eTMF in clinical trial research.
AQ
Industry News
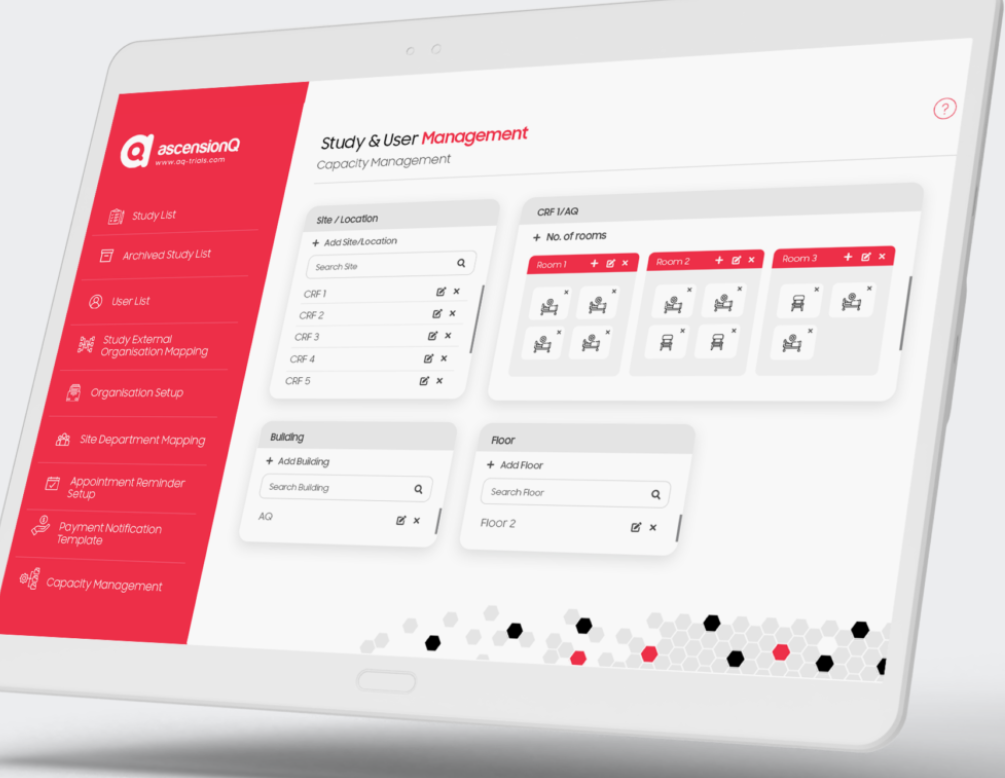
Navigating the World of CTMS and AscensionQ’s Clinical Operational Management System
Blog 08/09/2023
In the intricate domain of clinical research, Clinical Trial Management Systems (CTMS) have emerged as vital instruments. These platforms are the linchpin for orchestrating, monitoring, and administrating the diverse facets and data integral to clinical trials. As clinical research becomes increasingly multifaceted, CTMS has evolved from a mere tracking utility to a holistic solution that amalgamates various dimensions of clinical trials. This article aims to offer a thorough understanding of CTMS, its diverse components, and its role as a catch-all term. Additionally, we will shed light on AscensionQ’s innovative approach to clinical operational management, outlining its modules and features.

Industry News
Importance of AI in Clinical Trials from the Researchers’ Point of View
Blog 17/10/2023
The traditional methods of conducting and managing clinical trials are often time-consuming, expensive, and fraught with challenges. That’s why AI in clinical trials has become crucial. It holds the power to transform everything, effectively creating a bridge that connects scientific discoveries in the laboratory to real-world applications in healthcare. From diagnosing diseases to drug discovery, AI has significantly impacted the healthcare sector.

Industry News
Unveiling the Power of the Randomised Clinical Trial Study Design
Blog 13/08/2023
In the realm of medical research, the randomised clinical trial study design stands as a gold standard for assessing the efficacy and safety of interventions. This rigorous approach to experimentation offers invaluable insights into the effects of treatments, providing a solid foundation for evidence-based medicine. In this comprehensive guide, we’ll delve into the intricacies of the randomised clinical trial study design, while also exploring the distinctions between real-world studies and clinical trials. By frequently incorporating these essential keywords, we aim to bolster the SEO ranking and ensure that this vital information reaches those seeking a deeper understanding of research methodologies.

Industry News
Leveraging NHS Infrastructure for a Research-Active National Health Service
Blog 15/05/2023
The National Health Service (NHS) is a world-renowned healthcare system that not only provides vital healthcare services but also holds immense potential as a research powerhouse. With its extensive infrastructure, diverse patient population, and skilled healthcare professionals, the NHS is well-positioned to contribute significantly to medical research. In this blog post, we will explore how the NHS can leverage its infrastructure to become more research active, fostering innovation, improving patient outcomes, and driving scientific advancements.

Industry News
The Indispensable Role of a Clinical Trial Administrator in Successful Research Projects
Blog 25/06/2023
Clinical trials represent a crucial stage in medical research, offering hope for advancements in treatments and improved patient outcomes. While the spotlight often shines on researchers and physicians, there is another key player working tirelessly behind the scenes – the Clinical Trial Administrator. In this blog, we’ll explore the essential responsibilities and contributions of Clinical Trial Administrators in ensuring the smooth and efficient execution of research projects.

Industry News
The Clinical Trials Pharmacist: Safeguarding Compliance and Regulation with Electronic Pharmacy Files
Blog 28/06/2023
Clinical trials are vital for advancing medical research and developing innovative treatments for various diseases and conditions. In the complex world of clinical research, numerous professionals play critical roles in ensuring the trials’ success and safety. Among them, the Clinical Trials Pharmacist stands out as a key figure in handling medications and pharmaceutical aspects of the trials. In this blog, we will explore the importance of the Electronic Pharmacy File (EPF) and how Clinical Trials Pharmacists utilize it to maintain compliance and adhere to regulatory guidelines.

Industry News
The Thriving Landscape of Clinical Trials in Scotland: Glasgow at the Forefront
Blog 06/07/2023
Scotland has emerged as a vibrant hub for clinical research, attracting both national and international attention. With its robust healthcare infrastructure, world-class academic institutions, and supportive regulatory environment, Scotland offers a fertile ground for conducting cutting-edge clinical trials. Among the cities that shine brightly in this landscape, Glasgow stands at the forefront, playing a pivotal role in advancing medical research and shaping the future of healthcare. In this blog, we will explore the current state of clinical trials in Scotland, with a special focus on the opportunities and contributions from Glasgow.

Industry News
A Bright Horizon for Clinical Trials: Promising Future in London and Other Top Locations
Blog 09/07/2023
The world of clinical trials is on the cusp of a promising transformation, with cities across the UK leading the charge. While the COVID-19 pandemic brought challenges to the field, cities like London, Liverpool, Southampton, Bristol, Cardiff, and Nottingham are driving the resurgence of clinical research. In this blog, we will explore the exciting potential of these top locations, as they embrace innovation, collaboration, and patient-centred approaches to pave the way for a brighter future in clinical trials.

Industry News
Clinical Trial Supplies: A Vital Pillar for Successful Research
Blog 19/07/2023
In the realm of clinical trials, a key player often overlooked but indispensable for successful research is clinical trial supplies. This critical component forms the backbone of every study, ensuring the trial’s integrity, patient safety, and reliable research outcomes. As an expert in the field of clinical trials, the expert’s profound understanding of these supplies and the meticulous planning required to manage them effectively is evident. This blog will delve into the pivotal role of clinical trial supplies and the expert’s essential considerations, which contribute to the seamless integration of supplies into the research process.

Industry News
Clinical Trial Supply Services: Empowering Medical Advancements through Reliable Support
Blog 31/07/2023
Behind every successful clinical trial lies a well-orchestrated system of support that ensures the seamless flow of essential components – clinical trial supply services. These services form the backbone of research, ensuring the availability, quality, and timely delivery of critical supplies, including investigational drugs, placebos, medical devices, and ancillary materials. In this blog, we will explore the paramount importance of clinical trial supply services in the research process and the role they play in advancing medical science and improving patient outcomes.

